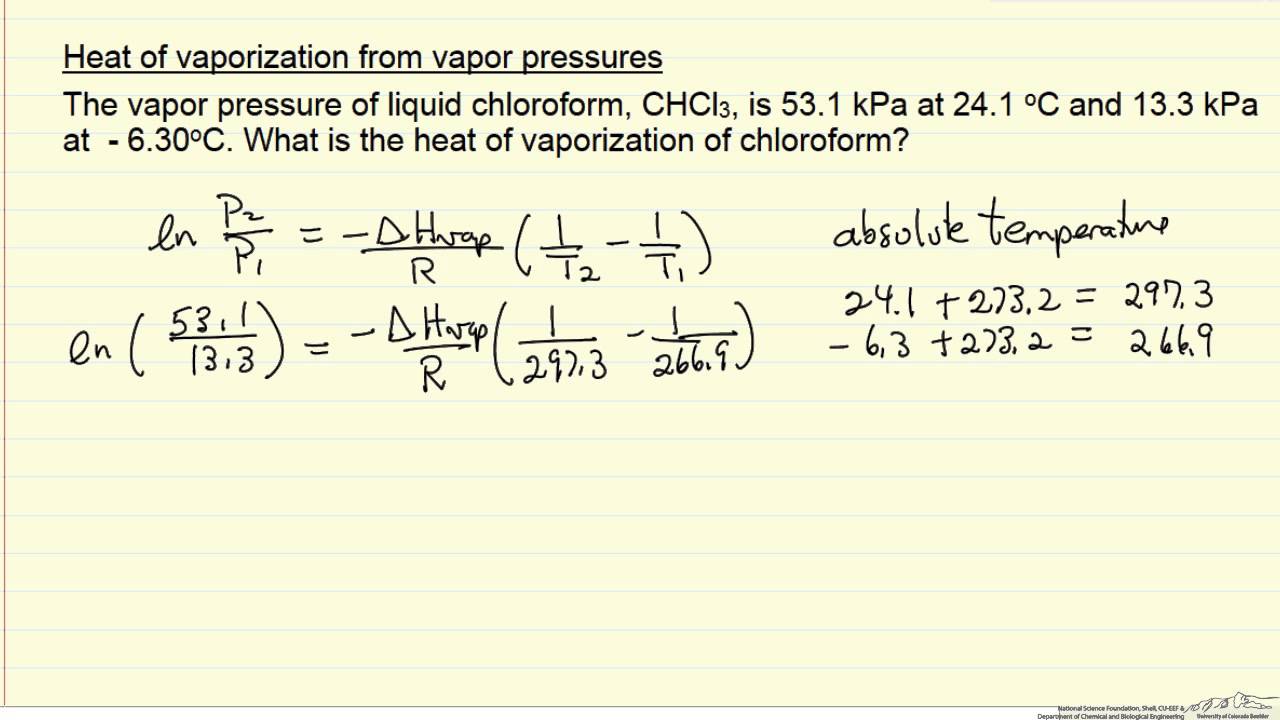
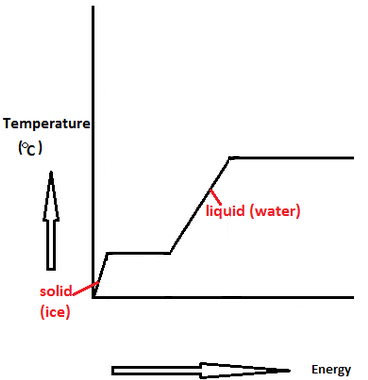
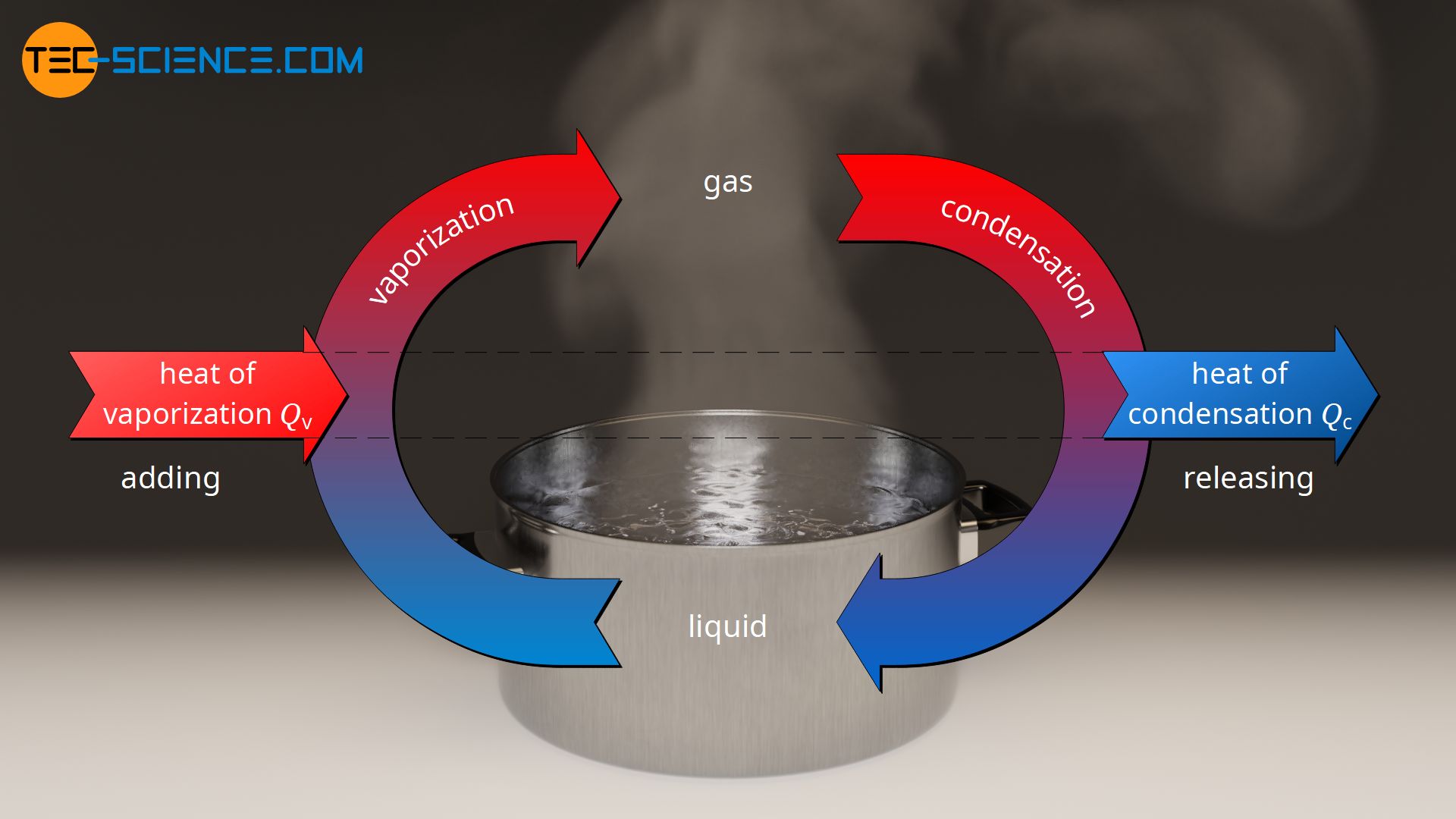
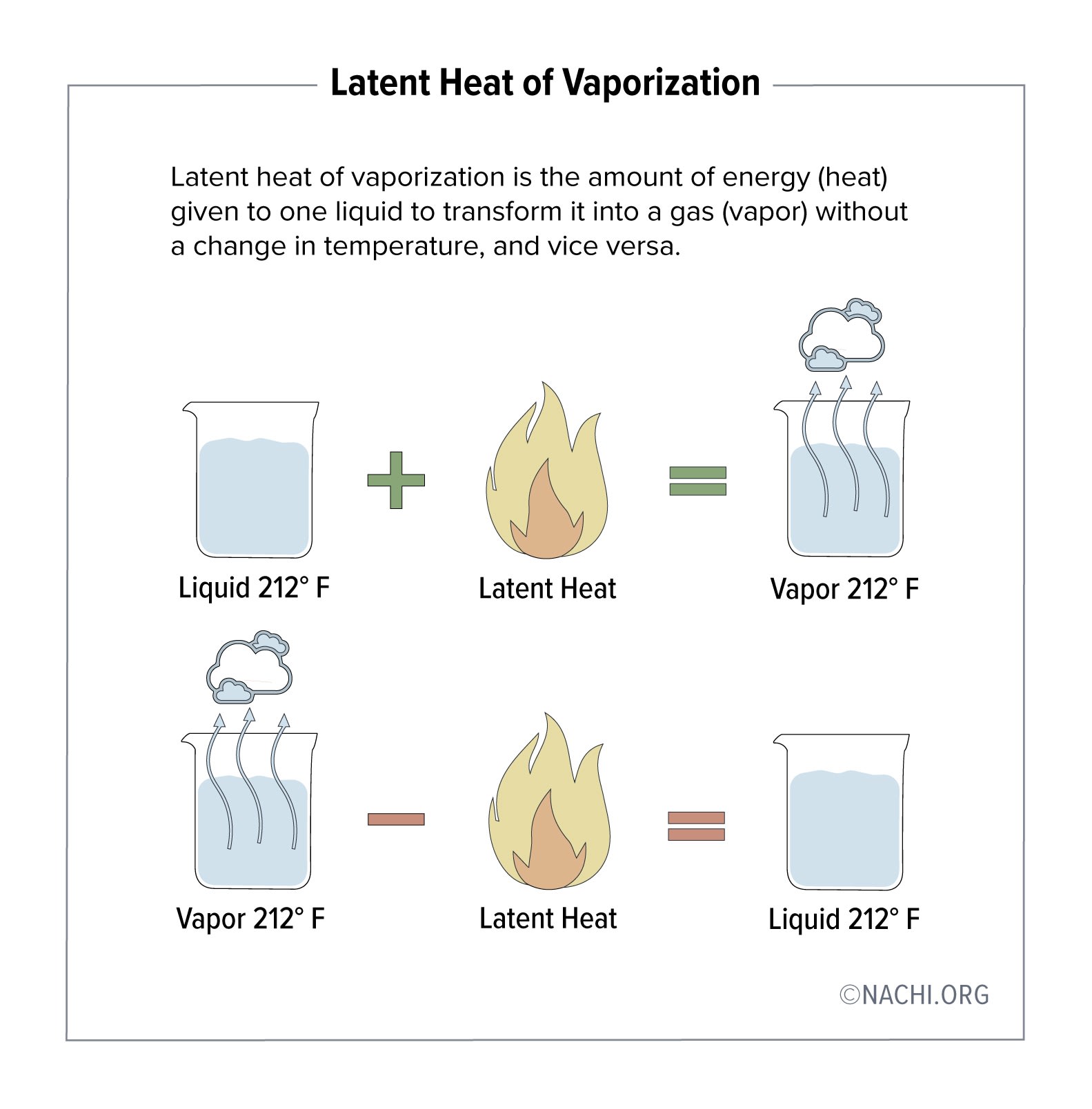
Does it take the same amount of energy to evaporate water completely over time as it takes to boil it completely (assume that you have the same amount of water)? - Quora

Gibbs free energy of vaporization ⌬ G vap ͑ P , T ͒ for TIP4P-Ew ͑ solid ͒ | Download Scientific Diagram
62 The latent heat of vaporization of a liquid at 500K and 1 atm pressure pressure is 20 Kcal/mol.what will be the change in internal energy of 3 mol of liquid at
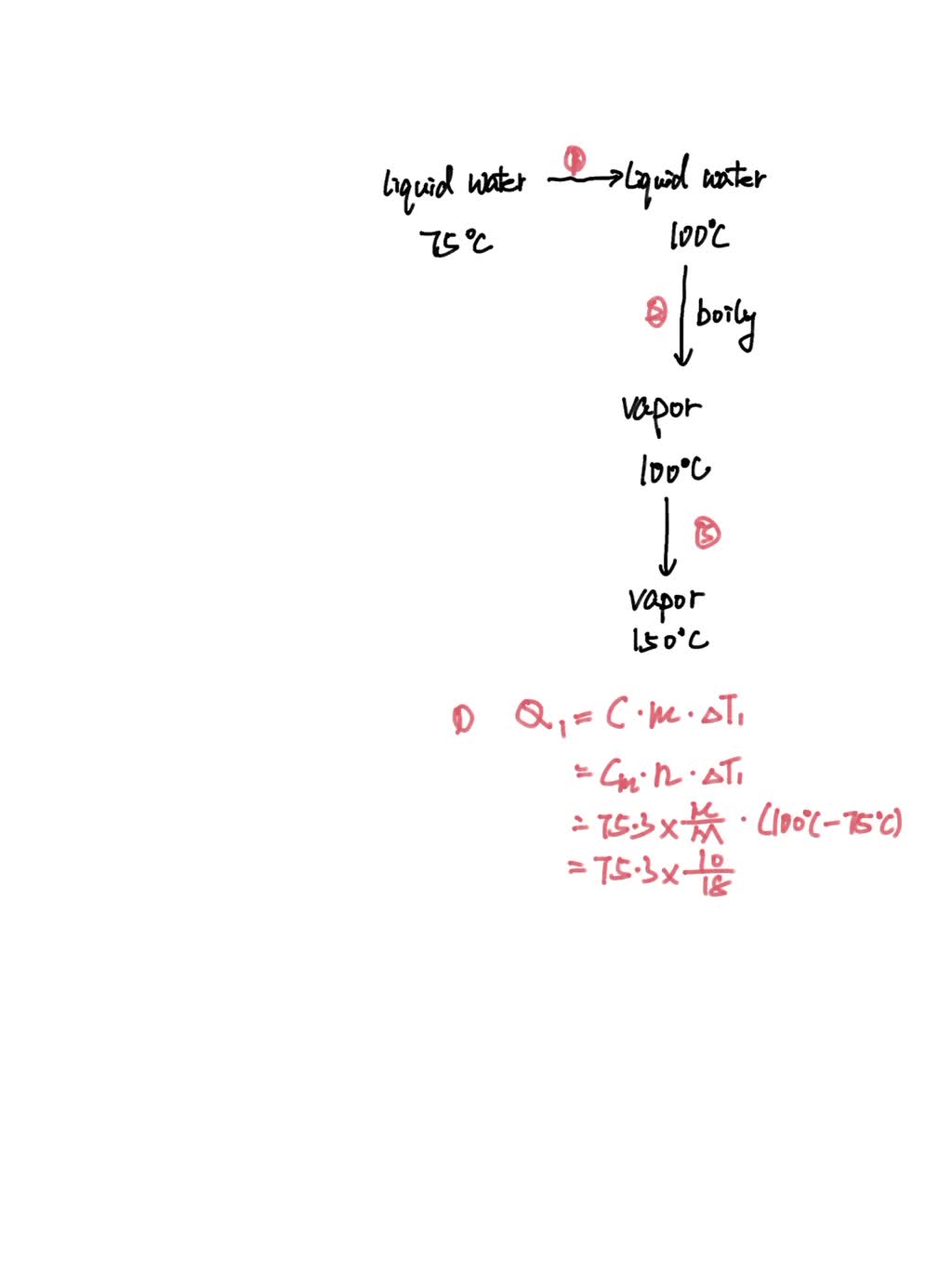
SOLVED: How much energy (in kilojoules) is absorbed when 10.0 g of liquid water at 75.0 °C is converted to water vapor at 150.0 °C? (The enthalpy of vaporization of water is

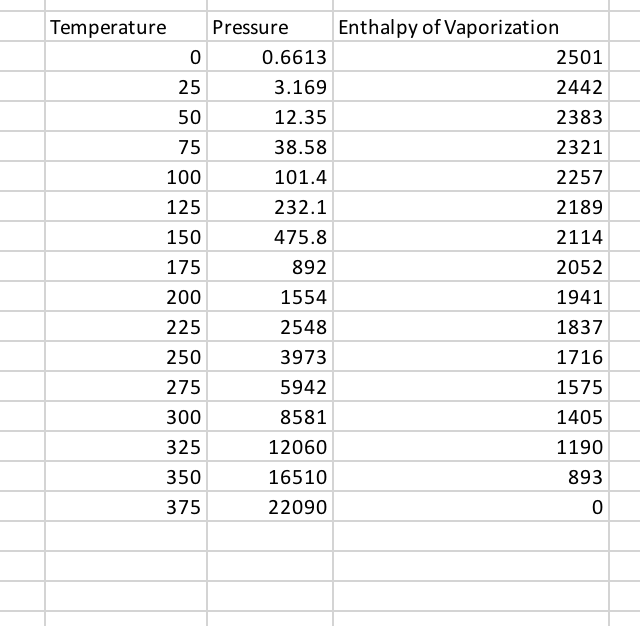
7a. The difference between the specific liquid and vapor enthalpy is... | Download Scientific Diagram